Armillarisin A
CAS No. 53696-74-5
Armillarisin A( —— )
Catalog No. M21210 CAS No. 53696-74-5
Armillarisin A enhancing the role of macrophages inhibiting bacteria growth improving the protein metabolism and regulating liver function.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 10MG | 43 | In Stock |


|
| 25MG | 69 | In Stock |


|
| 50MG | 97 | In Stock |


|
| 100MG | 144 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameArmillarisin A
-
NoteResearch use only, not for human use.
-
Brief DescriptionArmillarisin A enhancing the role of macrophages inhibiting bacteria growth improving the protein metabolism and regulating liver function.
-
DescriptionArmillarisin A enhancing the role of macrophages inhibiting bacteria growth improving the protein metabolism and regulating liver function.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number53696-74-5
-
Formula Weight234.2
-
Molecular FormulaC12H10O5
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (426.99 mM)
-
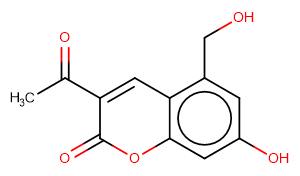
SMILESCC(C1=Cc(c(CO)cc(O)c2)c2OC1=O)=O
-
Chemical Name3-Acetyl-7-hydroxy-5-(hydroxymethyl)-2H-chromen-2-one
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.The Effects of Armillarisin A on Serum IL-1β and IL-4 and in Treating Ulcerative Colitis[J]. Cell Biochemistry & Biophysics 2015 72(1):103-106.
molnova catalog



related products
-
Menbutone
Menbutone is an organic compound with fomula C15H14O4.
-
15,26-Dihydroxylanos...
The fruit body of Ganoderma lucidum.
-
(2S)-3-Hydroxy-2-[4-...
The stems of Sambucus williamsii.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


